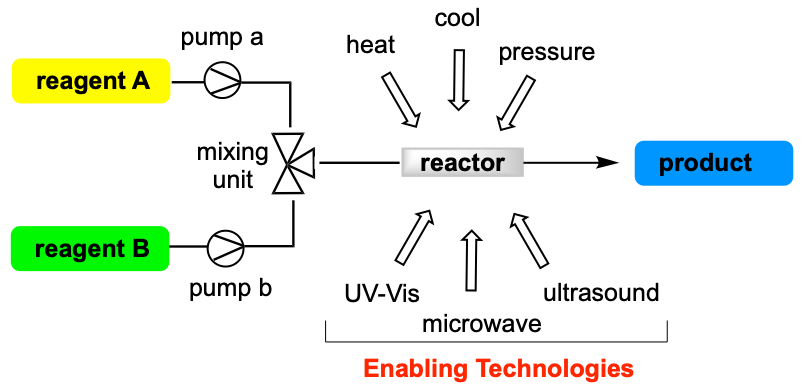
‘Flow Chemistry’ is today intended by the chemistry community as the set of operations (analysis and synthesis) performed in continuous flow regime in contrast to the conventional batch conditions. With particular reference to synthetic procedures, continuous-flow reactions offer several advantages toward process intensification, which can be summarized as follows:
- the easy scale-up (from laboratory to plant)
- the simple variation of process parameters (pressure, temperature, residence time) during the optimization phase
- easy automation
- the possible use of in-line supported reagents and scavengers as well as of extraction and analysis tools in multi-step synthesis;
- integration with enabling technologies (microwave, UV-Vis, ultrasound irradiation).
When synthetic flow processes are scaled-down in reactors with dimensions lower than one millimeter (Microreactor Technology, MRT), the hydrodynamic conditions of flowing fluids provide a favorable environment, which results in additional benefits in terms of chemical efficiency of the synthetic processes such as:
- controlled mixing of reactants by diffusion (laminar flow dominates)
- efficient mass transfer due to the favorable surface area-to-volume ratio for developing more selective syntheses
- efficient heat transfer with the possibility of working under superheating conditions
- easy management of poorly stable intermediates for safer transformations
- decreased inputs and (toxic) waste to improve processes sustainability
- enhanced photon-flux in photochemical reactions
- increased electrode surface-to-reactor volume ratio in electrochemical reactions
- improved solution-solid phase interactions in heterogenous continuous-flow reactions (Het-CFRs).
Reactions performed in packed-bed reactors with immobilized reagents and catalysts under continuous-flow conditions (Het-CFRs) are typically preferred to stirred batch reactions thanks to the less physical damage of the heterogenous support with potential long-term usage of the immobilized promoter (often a costly species) and the simplified reaction work-up, which only consists in the evaporation of the solvent. Additionally, telescoping multi-step reactions to one process in flow allows for the straightforward synthesis of complex molecules without the need of intermediate isolation; indeed, this approach is greatly facilitated by confining the immobilized reagent/catalyst in multiple fixed-bed reactors.
Additional basic concepts needed to practice flow chemistry concern the types of flow regimes and the knowledge of some definitions. The hydrodynamic flow (pressure-driven flow) is achieved by using common HPLC or syringe pumps and it is generated by a pressure difference between the inlet and outlet of the reactor. This strategy can be used for any liquid but the capillary resistance increases exponentially with decreasing the channel dimensions. The regime of electrodynamic flow is, instead, obtained by applying a potential bias between the inlet and outlet of the channel. Advantage of this approach is the precise fluid handling through the control of the applied voltage; an important limitation is, however, the compatibility with only polar solvents and glass- or silicon-based reactors.
The stoichiometry of a flow processes depends on the ratio of both flow rate and concentration of reagents that are fed into the reactor. The residence time of a flow process stands for the reaction time of a conventional batch reaction and it can be easily calculated from the reactor void volume of as follows:
Residence time (min) = reactor void volume (mL)/ flow rate (mL/min)
Selected literature
to start with flow chemistry
- “How to approach flow chemistry” Chem. Soc. Rev. 2020, 49, 8910-8932
- “Continuous flow chemistry: where are we now? Recent applications, challenges and limitations” Chem. Commun. 2018, 54, 13894-3928
- “Greener Approaches to Organic Synthesis Using Microreactor Technology” Chem. Rev. 2007, 107, 6, 2300-2318.
